|
Ice City Boyz, Skrapz
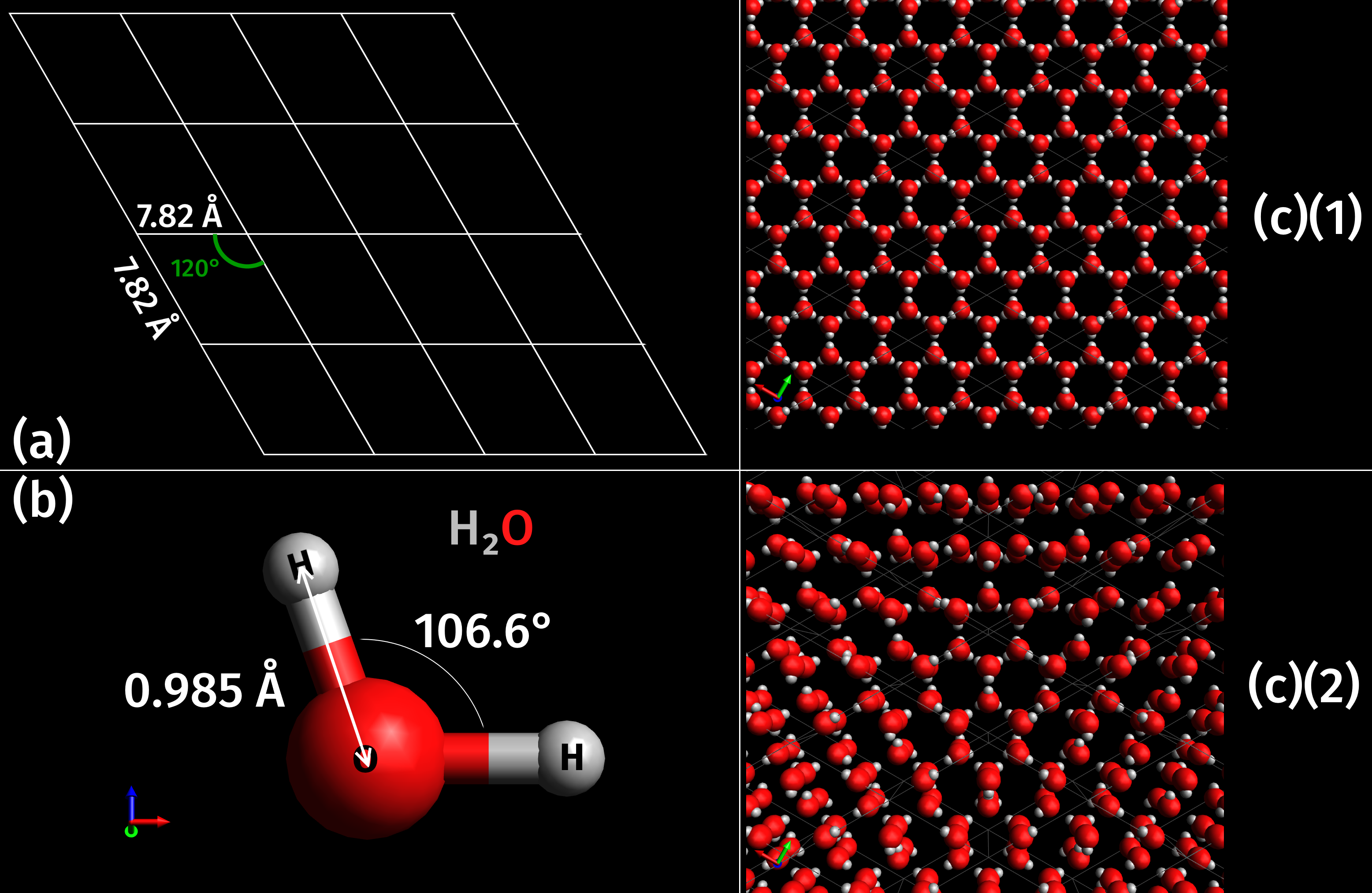
Ice is water that is frozen into a solid state, typically forming at or below temperatures of 0 ° C, 32 ° F, or 273.15 K. It occurs naturally on Earth, on other planets, in Oort cloud objects, and as interstellar ice. As a naturally occurring crystalline inorganic solid with an ordered structure, ice is considered to be a mineral. Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaque bluish-white color. Virtually all of the ice on Earth is of a hexagonal crystalline structure denoted as ''ice Ih'' (spoken as "ice one h"). Depending on temperature and pressure, at least nineteen phases ( packing geometries) can exist. The most common phase transition to ice Ih occurs when liquid water is cooled below (, ) at standard atmospheric pressure. When water is cooled rapidly (quenching), up to three types of amorphous ice can form. Interstellar ice is overwhelmingly low-density amorphous ice (LDA), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kilogram-force
The kilogram-force (kgf or kgF), or kilopond (kp, from ), is a non-standard Gravitational metric system, gravitational metric unit of force. It is not accepted for use with the International System of Units (SI) and is deprecated for most uses. The kilogram-force is equal to the magnitude of the force exerted on one kilogram of mass in a gravitational field (standard gravity, a conventional value approximating the average magnitude of gravity on Earth). That is, it is the weight of a kilogram under standard gravity. One kilogram-force is defined as .NIST]''Guide for the Use of the International System of Units (SI)''Special Publication 811, (1995) page 51 Similarly, a gram-force is , and a milligram-force is . History The gram-force and kilogram-force were never well-defined units until the CGPM adopted a ''standard acceleration of gravity'' of 9.80665 m/s2 for this purpose in 1901, though they had been used in low-precision measurements of force before that time. Even then, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Ice
Variations in pressure and temperature give rise to different phases of ice, which have varying properties and molecular geometries. Currently, twenty-one phases, including both crystalline and Amorphous solid, amorphous ices have been observed. In modern history, phases have been discovered through scientific research with various techniques including pressurization, force application, nucleation agents, and others. On Earth, most ice is found in the hexagonal Ice Ih phase. Less common phases may be found in the atmosphere and underground due to more extreme pressures and temperatures. Some phases are manufactured by humans for nano scale uses due to their properties. In space, amorphous ice is the most common form as confirmed by observation. Thus, it is theorized to be the most common phase in the universe. Various other phases could be found naturally in astronomical objects. Theory Most liquids under increased pressure freeze at ''higher'' temperatures because the pressu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deposition (phase Transition)
Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation and hence sometimes deposition is called desublimation. Applications Examples One example of deposition is the process by which, in sub-freezing air, water vapour changes directly to ice without first becoming a liquid Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th .... This is how frost and hoar frost form on the ground or other surfaces, including leaves. For deposition to occur, thermal energy must be removed from a gas. When the air becomes cold enough, water vapour in the air surrounding a leaf loses enough thermal energy to change into a solid. Even though the air tempera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
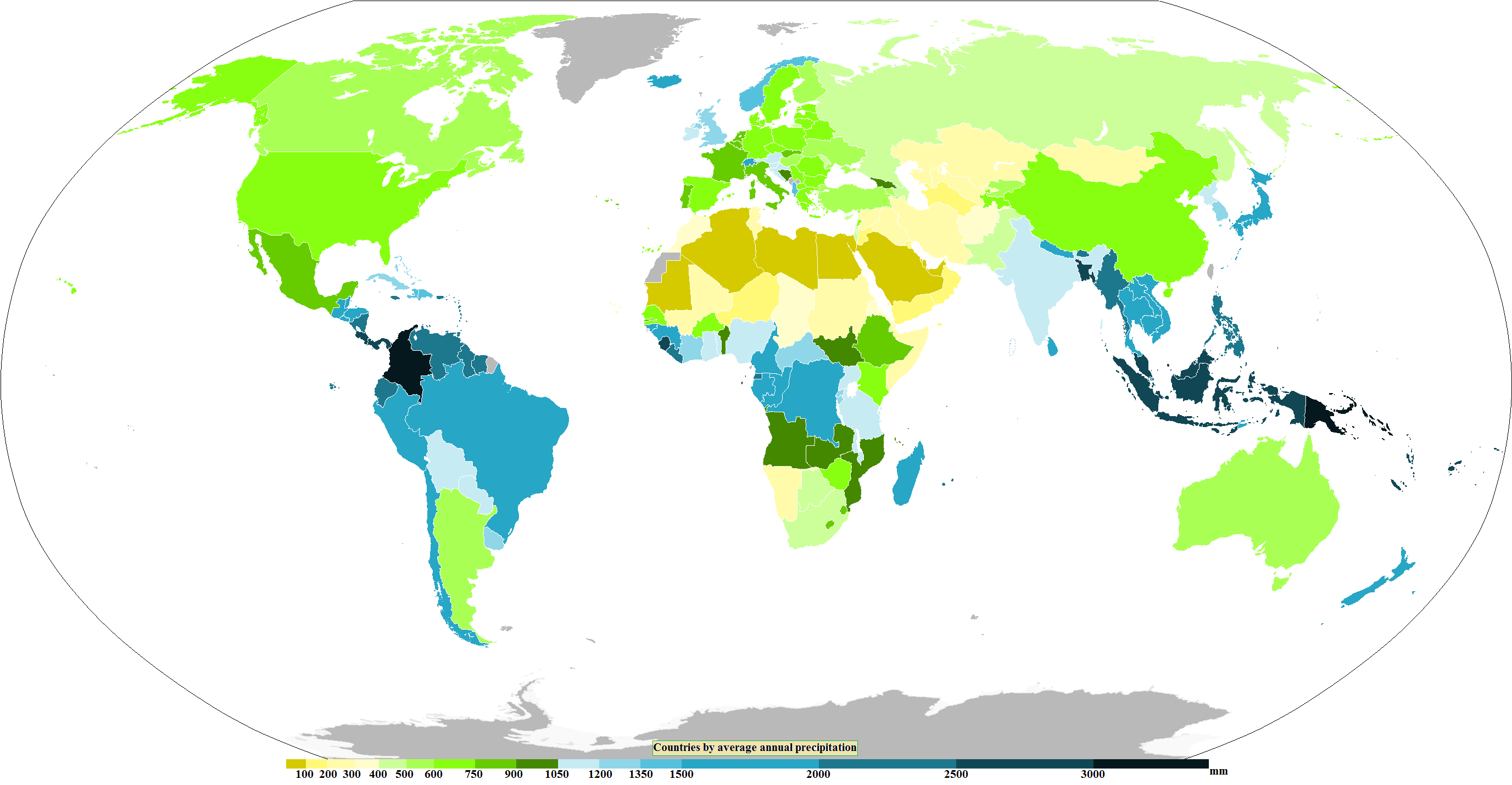
Precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwealth usage), snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. (Such a non-precipitating combination is a colloid.) Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hail
Hail is a form of solid Precipitation (meteorology), precipitation. It is distinct from ice pellets (American English "sleet"), though the two are often confused. It consists of balls or irregular lumps of ice, each of which is called a hailstone. Ice pellets generally fall in cold weather, while hail growth is greatly inhibited during low surface temperatures. Unlike other forms of ice, water ice precipitation, such as graupel (which is made of rime ice), ice pellets (which are smaller and Transparency and translucency, translucent), and snow (which consists of tiny, delicately crystalline flakes or needles), hailstones usually measure between and in diameter. The METAR reporting code for hail or greater is GR, while smaller hailstones and graupel are coded GS. Hail is possible during most thunderstorms (as it is produced by cumulonimbus), as well as within of the parent storm. Hail formation requires environments of strong, upward motion of air within the parent thunderst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Snowflakes
A snowflake is a single ice crystal that is large enough to fall through the Earth's atmosphere as snow.Knight, C.; Knight, N. (1973). Snow crystals. Scientific American, vol. 228, no. 1, pp. 100–107.Hobbs, P.V. 1974. Ice Physics. Oxford: Clarendon Press. Snow appears white in color despite being made of clear ice. This is because the many small crystal facets of the snowflakes scatter the sunlight between them. Each flake begins by forming around a tiny particle, called its nucleus, accumulating water droplets, which freeze and slowly form a crystal. Complex shapes emerge as the flake moves through differing temperature and humidity zones in the atmosphere, and possibly combines with other snowflakes. Because of this, snowflakes tend to look very different from one another. However, they may be categorized in eight broad classifications and at least 80 individual variants. The main constituent shapes for ice crystals, from which combinations may occur, are ''needle'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
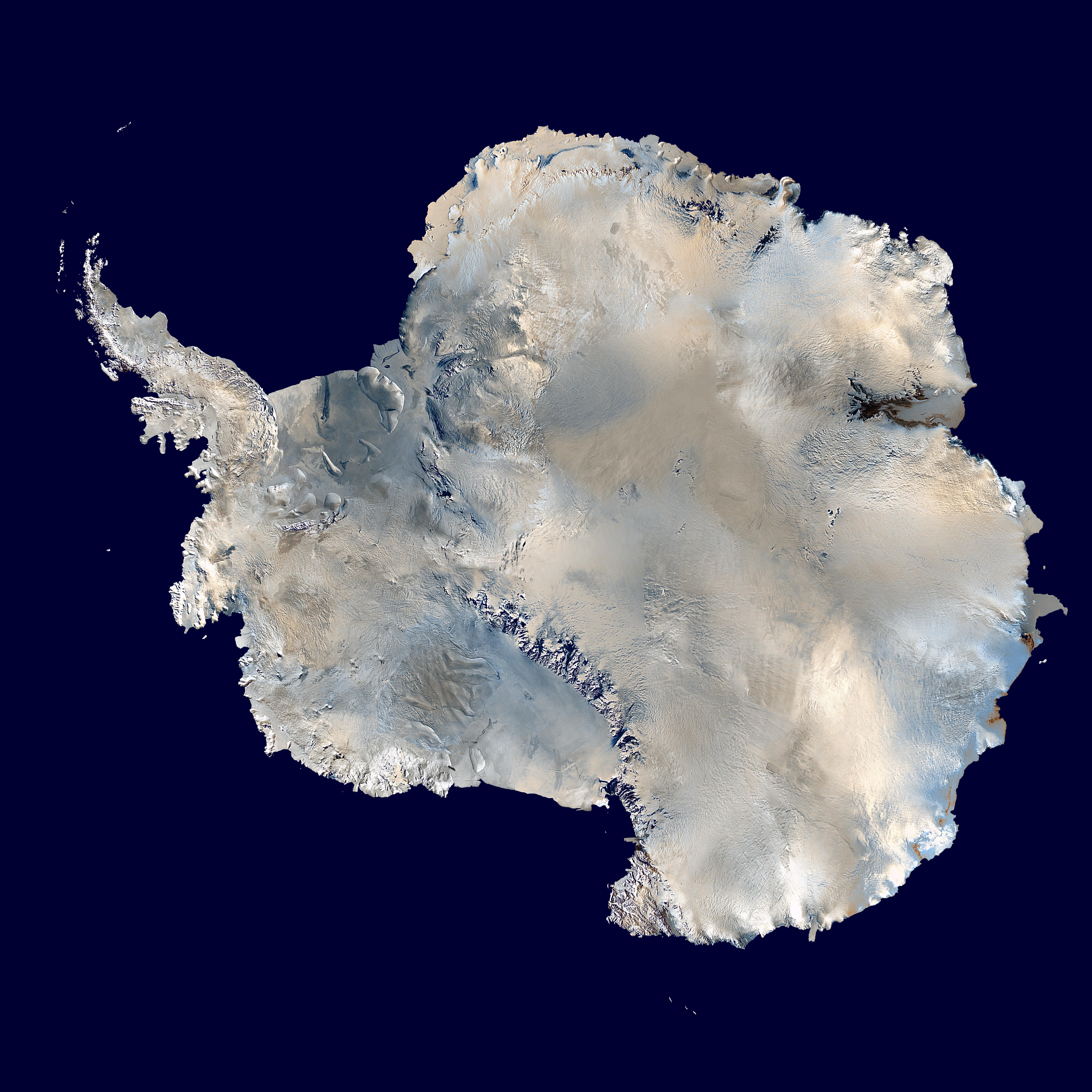
Ice Sheet
In glaciology, an ice sheet, also known as a continental glacier, is a mass of glacier, glacial ice that covers surrounding terrain and is greater than . The only current ice sheets are the Antarctic ice sheet and the Greenland ice sheet. Ice sheets are bigger than ice shelf, ice shelves or alpine glaciers. Masses of ice covering less than 50,000 km2 are termed an ice cap. An ice cap will typically feed a series of glaciers around its periphery. Although the surface is cold, the base of an ice sheet is generally warmer due to Geothermal activity, geothermal heat. In places, melting occurs and the melt-water lubricates the ice sheet so that it flows more rapidly. This process produces fast-flowing channels in the ice sheet — these are ice streams. Even stable ice sheets are continually in motion as the ice gradually flows outward from the central plateau, which is the tallest point of the ice sheet, and towards the margins. The ice sheet slope is low around the plate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |